Research
Interactions of Hsp70 and Apolipoprotein E with amyloid proteins
Chaperone protein Hsp70: Heat shock protein (Hsp70) is ubiquitously expressed. It plays important roles preventing misfolding and aggregation of proteins in the intracellular environment. We are investigating how Hsp70 interacts with IAPP and alpha-synuclein to prevent amyloid aggregation.
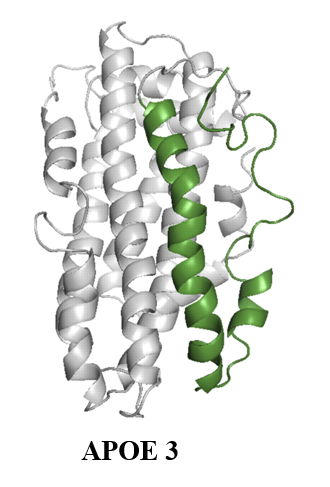
Apolipoprotein E (ApoE): ApoE is a major lipoprotein in the brain. It plays crucial roles in maintaining neuronal health. However, E4 isoform of apoE is the strongest risk factor of late onset Alzheimer’s disease. We are using single molecule techniques to study the role of apoE on inhibition or promotion of aggregation of Aβ peptides.
Biomolecular condensation and Liquid-liquid Phase Separation (LLPS)
Liquid-liquid phase separation (LLPS) of proteins and RNA molecules is responsible for formation of membrane-less organelles such as the nucleoli and the PML bodies in the nucleus, and the stress granules in the cytoplasm. Beyond compartmentalization the biomolecular condensates have been found to be crucial in biological functions such as transcription, nuclear transport and signalling. We are interested in understanding the molecular mechanism of biomolecular condensation such as liquid-liquid phase separation of proteins and nucleic acids leading to formation of membrane-less organelles with specialized functions, and amyloid aggregation that are involved in the pathology of multiple degenerative diseases.
Currently, we are investigating phase diagram of the LLPS of Aβ, α-synuclein and tau proteins.
Single Molecule Techniques
We build single molecule biophysical techniques to study aggregation, phase separation and biomolecular interactions. The major techniques built in our lab are
- Fluorescence Correlation Spectroscopy (FCS) and Single Molecule FRET (smFRET): The home-built FCS is equipped multiple lasers and multiple detection channels. Hence, it can perform both auto- and cross-correlation FCS. We can also use Pulsed Interleaved Excitation (PIE) to eliminate spectral cross-talk, which is important in accurate measurements of smFRET and cross-correlation FCS.
- Cuvette-FCS: We have developed a novel FCS technique that can perform FCS measurements inside cuvettes. It can be used to perform FCS measurements in extreme conditions such high or low temperature or pressure, and in harsh solvents.
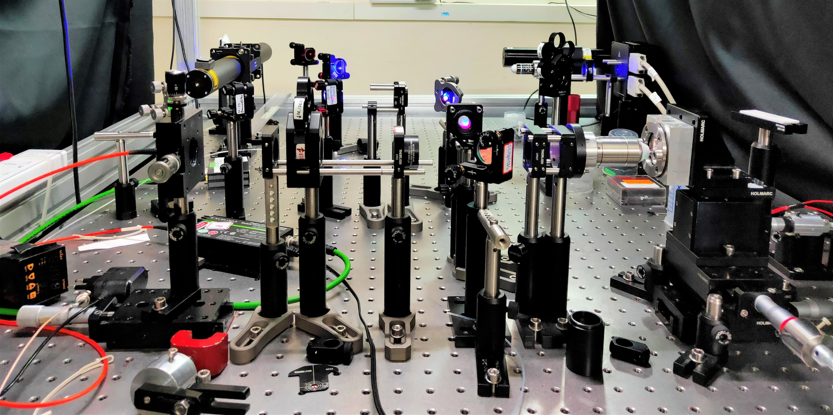
Sahoo, BJ 2018 and Sil, Methods in Enzymology 2018
Super-resolution Optical Microscopy
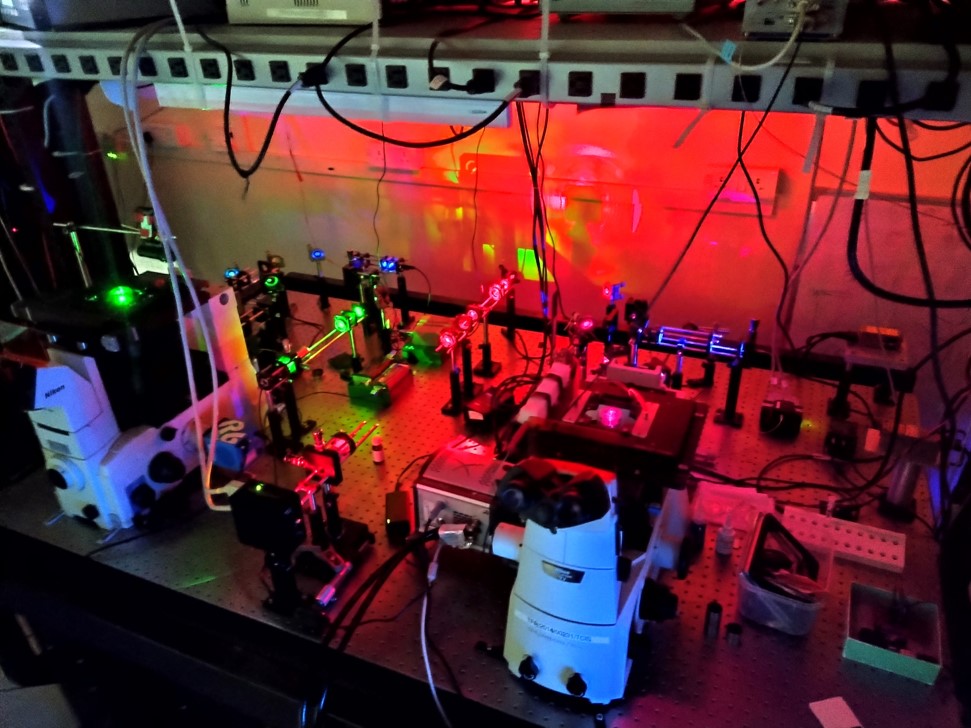
We have built a Total Internal Reflection Fluorescence Microscope (TIRFM), which can perform super-resolution optical microscopy such as STORM. This microscope is also equipped with accessories for performing FCS and FRAP experiments.
DIY Instrumentation
In addition to the above-mentioned techniques, we are continuously building various biophysical instruments of high quality but at very low cost. These techniques include home-built spectrofluorometer, lasers and detectors.
