#PhDone: Studying protein conformational dynamics over large timescales
Congratulations to Nihar Khandave on successfully defending his thesis titled ‘Extending CEST NMR experiments to study protein conformational dynamics occurring over the ~10-4 to ~100 seconds timescale’.
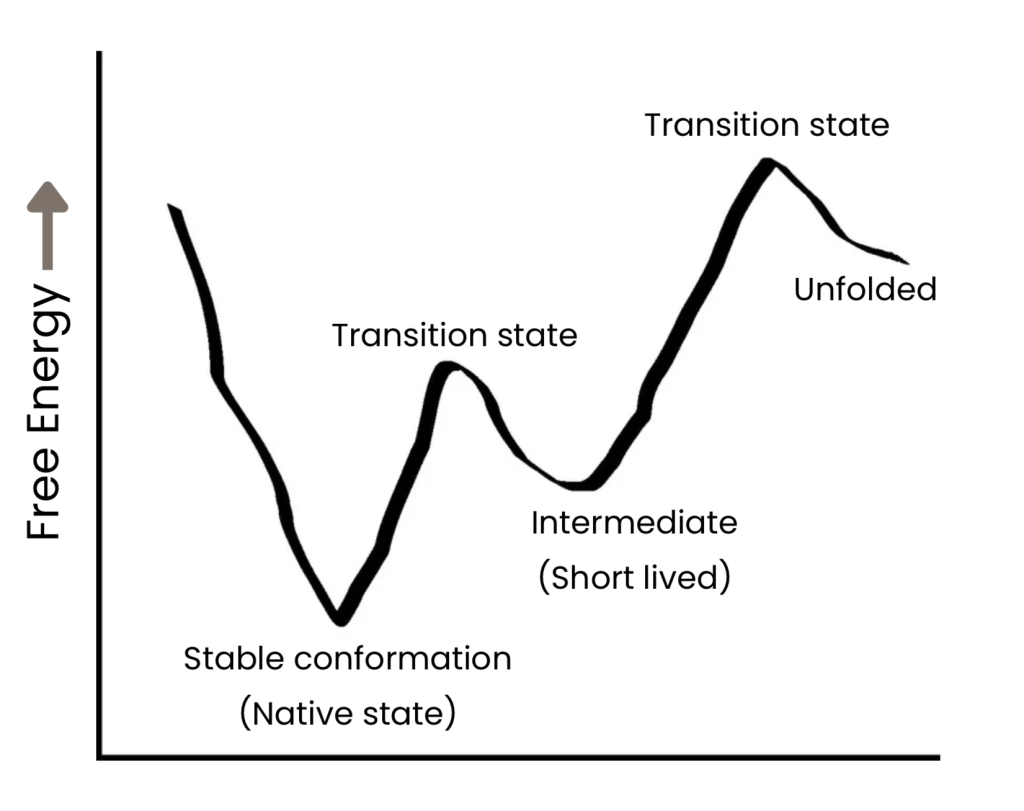
Proteins can exist in multiple three-dimensional conformations. Often, the most stable native conformation is prevalent while less populated ‘excited’ states exist in equilibrium with native state and each other. The protein molecule has to switch between these different conformations to perform various tasks (such as binding to ligands like a drug molecule or interaction with other proteins etc.). Understanding the underlying dynamics between the native conformation and excited conformation(s) can help provide crucial insights into protein activity and folding.
As far as conformational dynamics is concerned, these excited transient states are often short-lived with lifetimes from microseconds (~1000000 exchange events in a second; fast dynamics) to seconds (~1 exchange event in a second; slow dynamics).
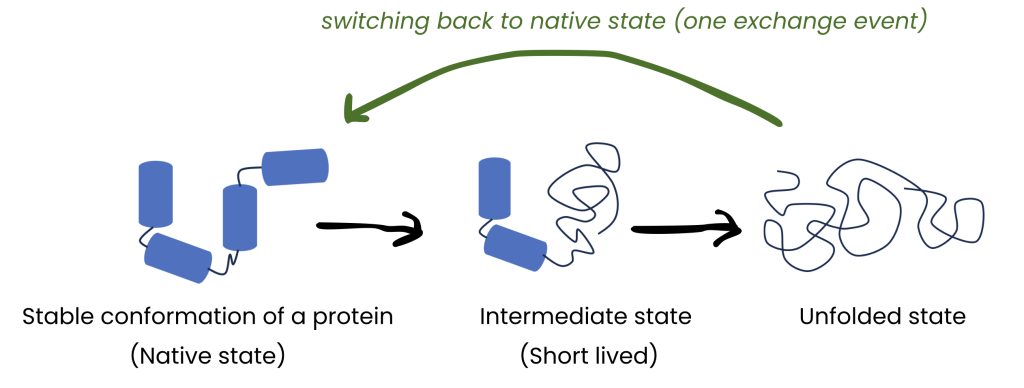
How transient are these excited states?
– Short lifetimes of ‘excited’ states: ‘~1000000 exchange events in a second’ = The excited state changes into the native state and back (in a continuous cycle) for approximate 1000000 times in a second.
– Long(er) lifetimes: ‘~1 exchange event in a second’ = The excited state changes into the native state and back once in a second.
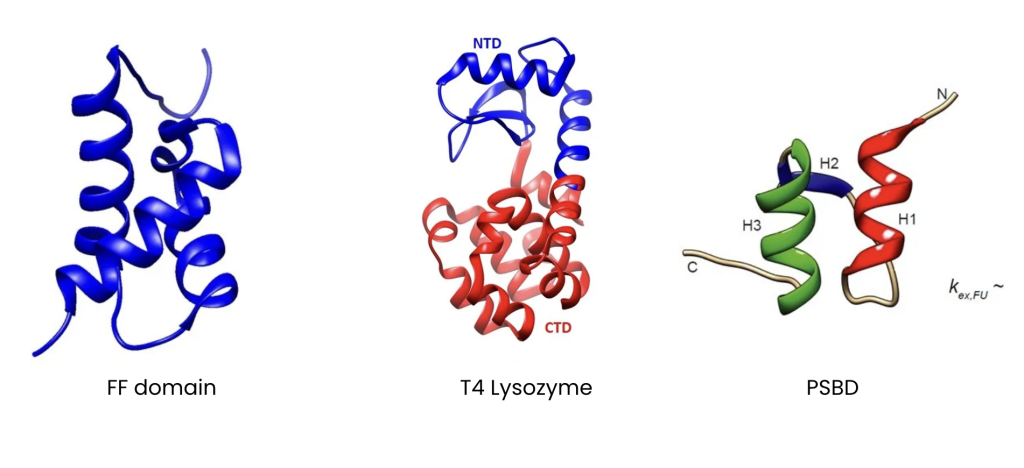
Due to the transient nature of the excited states, they are not directly observable through conventional NMR experiments (or conventional biophysical techniques) and therefore need specialized NMR experiments. Typically, different NMR experiments are used for studying slow, intermediate and fast conformational dynamics, but here Nihar shows that the Chemical Exchange Saturation Transfer (CEST) NMR experiments can be used to study protein conformational dynamics spanning a broad range of timescale (1 to 10,000 exchange events per second). He demonstrates this process to observe transient populations of ‘excited’ conformations in three proteins: FF domain, T4 Lysozyme and the peripheral subunit binding domain (PSBD).
Read more about Nihar’s work at:
– Using the amide 15N CEST NMR experiment to study slow exchange between ‘visible’ protein states
– Studying micro to millisecond protein dynamics using simple amide 15N CEST experiments supplemented with major-state R2 and visible peak-position constraints
