Disorder, misfolding, and aggregation: Simulating how different microenviroments affect alpha-synuclein
Strands of amino acids linked together with peptide bonds, carefully fold to form three dimensional protein structures. These structures determine the function of a specific protein. A class of proteins called ‘intrinsically disordered proteins’ (IDPs) are an anomaly to this phenomenon and do not fold into one distinct 3D structure. Despite their lack of a well defined structure, multiple studies have revealed that these proteins do in fact play a range of roles in the cellular processes.
An example of such an intrinsically disordered protein is alpha-synuclein which is found in the synapses of our neurons. While it is indicative that it may play a role in neurotransmission, its true physiological role remains unknown. What has come to researchers’ attention, however, is how this protein forms aggregates and is a hallmark pathological feature in the neurons of Parkinson’s patients. Abdul Wasim, during his PhD days in TIFR Hyderabad, took a closer look into the possible factors that contribute to the aggregation of these proteins.
A homogenous solution may sometimes spontaneously separate out into high and low density regions of its solute; this phenomenon is called Liquid Liquid Phase Separation (LLPS). Alpha-synuclein, a protein initially present in solution with other proteins in the brain, undergoes LLPS to form aggregates. While LLPS is reversible, it could be a precursor to more stable aggregates that are key in pathological conditions as they may hamper normal cellular function, in this case – synaptic transmission.
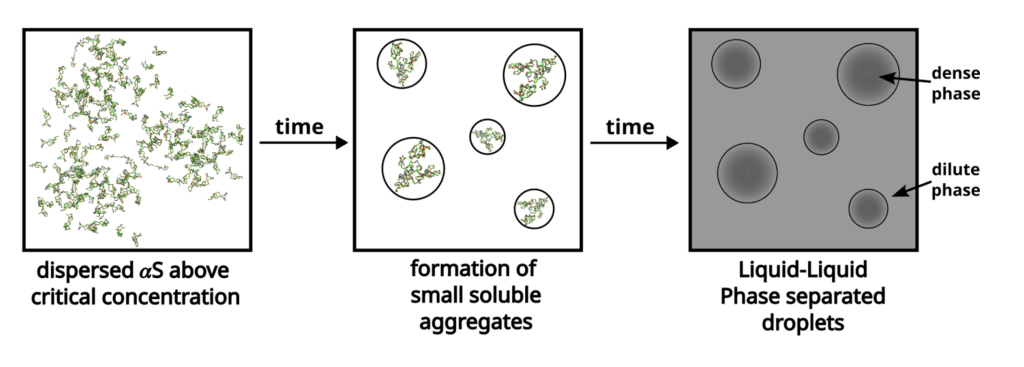
A schematic showcasing the process of liquid-liquid phase separation of α-synuclein (αS).
From Wasim et al., “Modulation of α-synuclein aggregation amid diverse environmental perturbation,” eLife, 2024. (CC BY 4.0)
Knowing how aggregates form makes it very crucial for us to understand the environmental factors that are causing this misfolding of alpha-synuclein, triggering it to undergo LLPS and form aggregates. Abdul Wasim, a computational biologist, simulated how the protein would aggregate upon slight changes in the physiological environment of the neuron synapse.
In one instance, the behaviour of alpha-synuclein is observed in water. Wasim saw that while aggregate formation did occur, most protein chains remained free and water itself was not promoting the aggregation. Next, Wasim put this protein in salts (NaCl + water). In this condition, alpha-synuclein showed an increased propensity towards aggregation, notably higher than the previous one. Finally, he observed what happens when this protein is put amongst crowders (that mimics other biomolecules present in the environment). The propensity to aggregate was higher this time too! However, in an interesting observation, Wasim found that the latter two conditions (salt and crowders) promote LLPS through different mechanisms: while the salt increased surface tension, the crowders caused an increased relative concentration of alpha-synuclein.
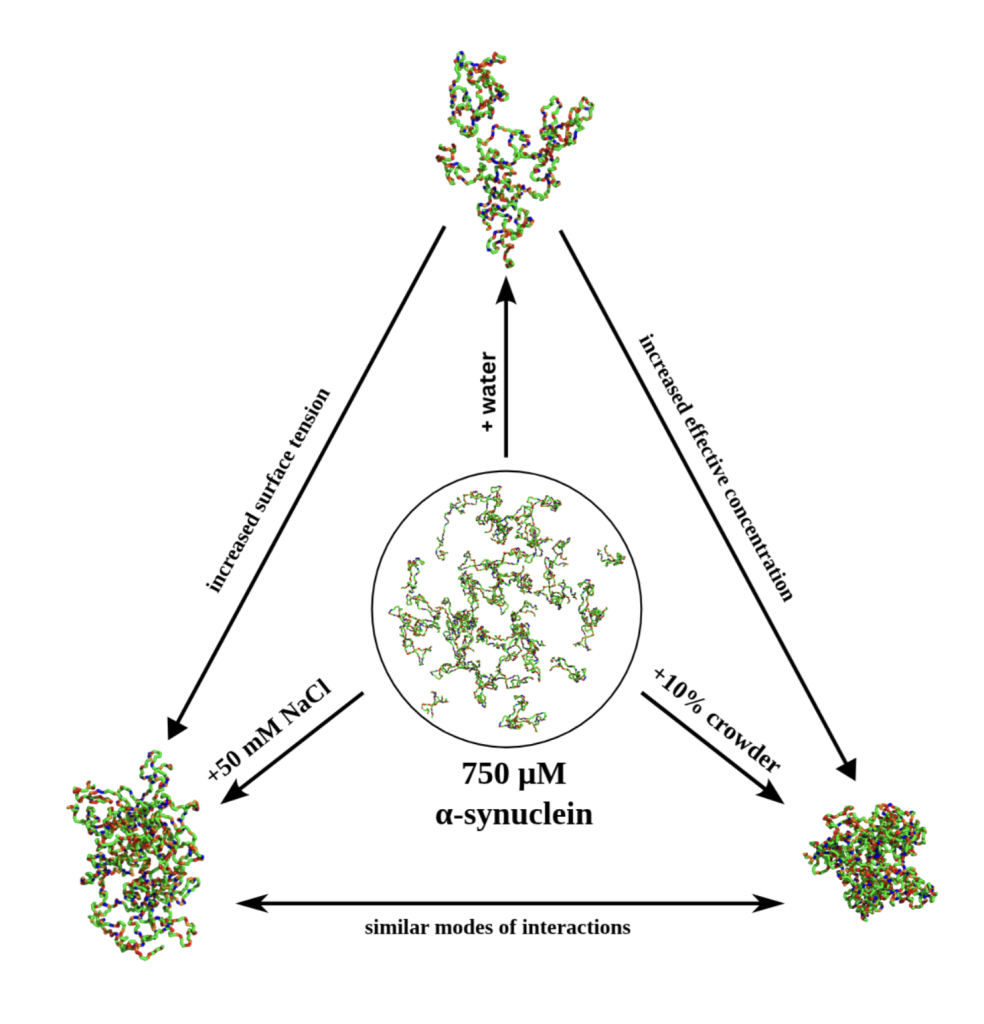
Graphical abstract adapted from Wasim et al. eLife 2024. (CC BY 4.0)
Wasim further looked out for hallmarks of Liquid Liquid Phase Separation in the alpha-synuclein aggregates which merged together to alleviate surface tension. The molecules also extend its shape to enable favourable interactions and aggregate formation.
Wasim also observed one alpha-synuclein chain’s interaction with other proteins in its proximity. While some interactions supported or even promoted aggregate formation, some others stopped the aggregation from taking place. This indicates the existence of a cellular mechanism that prevents aggregation in normal conditions.
These simulations were based on experiments conducted by Soumik Ray and colleagues from IIT Bombay. Their studies were published in Nature Chemistry in 2020.
Wasim and colleagues hope that these insights on alpha-synclein aggregation would lead to a better understanding of the physiological role of the protein. Further, this study takes a closer look at how the immediate microenvironment around alpha-synuclein may contribute to its aggregation, and what could perhaps help alleviate this aggregate formation.
Content: Srushti Chipde, Editor: Anusheela Chatterjee
With reviews and inputs from: Abdul Wasim
